Balancing Equations Chemistry Worksheet Answer Key
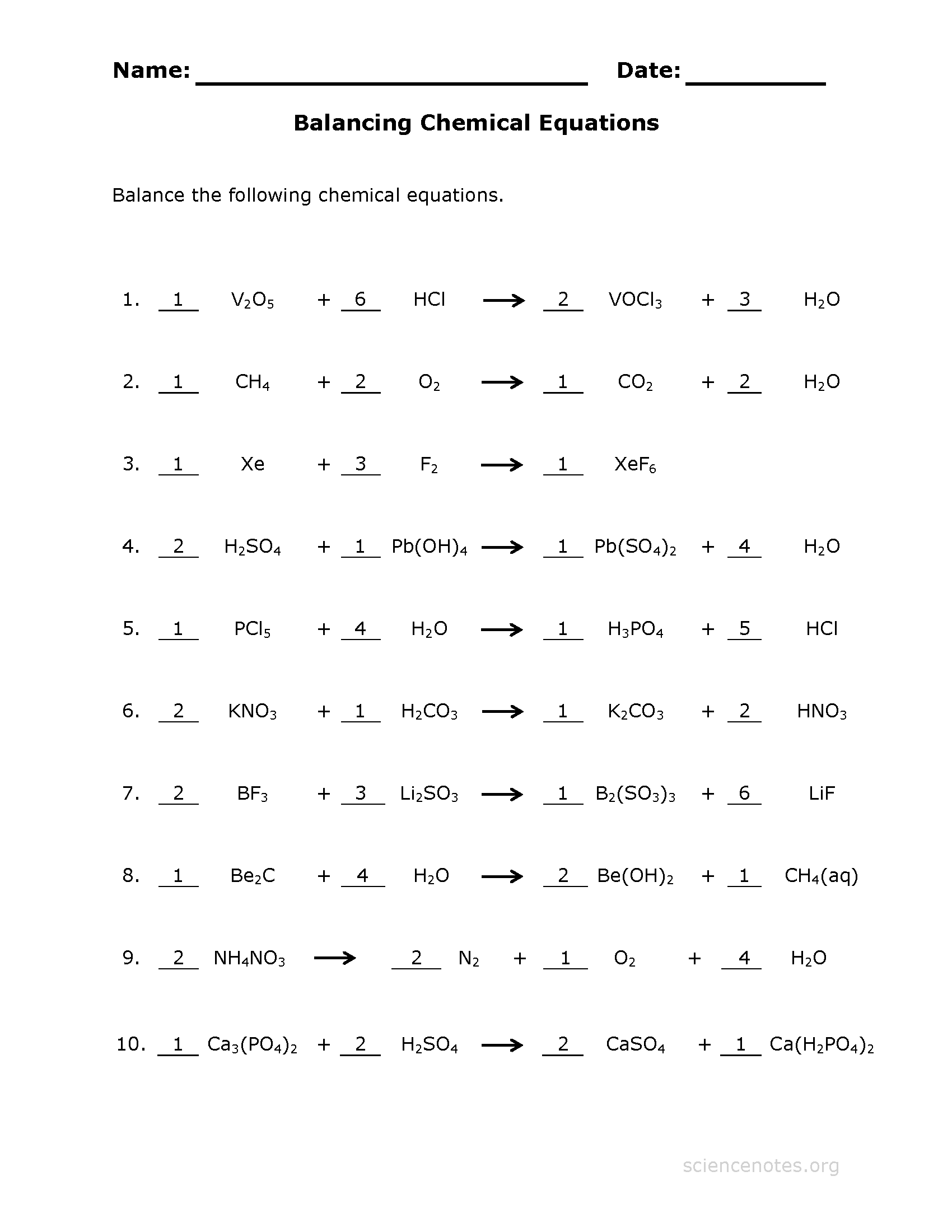
Balancing Equations Chemistry Worksheet Answer Key - Label the chemical equation using product, reactants, subscript, coefficient, and yields. (1) circle each subscript in each chemical formula. First, balance each of the chemical equations below. 1) 1 n 2 + 3 h 2 æ 2 nh 3 2) 2 kclo 3 æ 2 kcl + 3 o 2 3) 2 nacl + 1 f 2 æ 2 naf + 1 cl 2 4) 2 h 2 + 1 o 2 æ 2 h 2o 5) 1 pb(oh) 2 + 2 hcl æ 2 h 2o + 1 pbcl 2 6) 2 albr 3 + 3 k 2so 4 æ 6 kbr + 1 al 2(so. 1) 1 n2 + 3 h2 æ 2 nh3 2) 2 kclo3 æ 2 kcl + 3 o2 3) 2 nacl + 1 f2 æ 2 naf + 1 cl2 4) 2 h2 + 1 o2 æ 2 h2o 5) 1 pb(oh)2 + 2 hcl æ 2 h2o + 1 pbcl2 6) 2 albr3 + 3 k2so4 æ 6 kbr + 1 al2(so4)3 7) 1 ch4. 2 h 2 + 1 o 2 2 h 2 o;
2 albr 3 + 3 k 2. (3) answer the questions related to each chemical formula. 2 kclo 3 2 kcl + 3 o 2; Balancing equations practice answer key part a: First, balance each of the chemical equations below.
2 h 2 + 1 o 2 2 h 2 o; Up to 24% cash back more chemistry tutorials and practice can be found at www.chemfiesta.com. Identify the following parts of each chemical formula by circling the subscripts and drawing a square around the coefficients. 1 ch 4 + 2 o 2 2 nacl + 1 f 2 2 naf.
1 ch 4 + 2 o 2 To earn full credit, write the words out when classifying. (1) circle each subscript in each chemical formula. 1) 1 n 2 + 3 h 2 2 nh 3 2) 2 kclo 3 2 kcl + 3 o 2 3) 2 nacl + 1 f 2 2 naf + 1 cl 2 4).
2 kclo 3 2 kcl + 3 o 2; Label the chemical equation using product, reactants, subscript, coefficient, and yields. 2 nacl + 1 f 2 2 naf + 1 cl 2; 1 ch 4 + 2 o 2 To earn full credit, write the words out when classifying.
1 ch 4 + 2 o 2 First, balance each of the chemical equations below. Write the word equations below as chemical equations and balance: 2 h 2 + 1 o 2 2 h 2 o; Identify the following parts of each chemical formula by circling the subscripts and drawing a square around the coefficients.
1 pb(oh) 2 + 2 hcl 2 h 2 o + 1 pbcl 2; Write the word equations below as chemical equations and balance: (1) circle each subscript in each chemical formula. 1 n 2 + 3 h 2 2 nh 3; Label the chemical equation using product, reactants, subscript, coefficient, and yields.
Balancing Equations Chemistry Worksheet Answer Key - 2 albr 3 + 3 k 2. 2) aluminum bromide and chlorine gas react to form aluminum chloride and bromine gas. (2) draw a square around each coefficient. Write the word equations below as chemical equations and balance: Balancing equations practice answer key part a: (1) circle each subscript in each chemical formula.
(1) circle each subscript in each chemical formula. 2 kclo 3 2 kcl + 3 o 2; 1) _1_ h 3po 4 + _3__ koh æ _1__ k 3po 4 + _3__ h 2o. 1 n 2 + 3 h 2 2 nh 3; 2 fe + 3 h.
Writing And Balancing Equations Worksheet.
2 albr 3 + 3 k 2. 2 nacl + 1 f 2 2 naf + 1 cl 2; Balancing equations answers 1) 1 al(no 3) 3 + 1 (nh 4) 3po 4 → 1 alpo 4 + 3 nh 4no 3 2) 2 agf + 1 cacl 2 → 2 agcl + 1 caf 2 3) 1 znbr 2 + 1 pb(no 2) 2 → 1. (1) circle each subscript in each chemical formula.
Balancing Equations Practice Answer Key Part A:
1 fe 2(so 4) 3 + 3 h 2 2. 2 h 2 + 1 o 2 2 h 2 o; 1) 1 n2 + 3 h2 æ 2 nh3 2) 2 kclo3 æ 2 kcl + 3 o2 3) 2 nacl + 1 f2 æ 2 naf + 1 cl2 4) 2 h2 + 1 o2 æ 2 h2o 5) 1 pb(oh)2 + 2 hcl æ 2 h2o + 1 pbcl2 6) 2 albr3 + 3 k2so4 æ 6 kbr + 1 al2(so4)3 7) 1 ch4. Balance the following chemical equations.
To Earn Full Credit, Write The Words Out When Classifying.
(2) draw a square around each coefficient. Balance the following chemical equations. 1) _1_ h 3po 4 + _3__ koh æ _1__ k 3po 4 + _3__ h 2o. Identify the following parts of each chemical formula by circling the subscripts and drawing a square around the coefficients.
Up To 24% Cash Back More Chemistry Tutorials And Practice Can Be Found At Www.chemfiesta.com.
1) 1 n2 + 3 h2 æ 2 nh3 2) 2 kclo3 æ 2 kcl + 3 o2 3) 2 nacl + 1 f2 æ 2 naf + 1 cl2 4) 2 h2 + 1 o2 æ 2 h2o 5) 1 pb(oh)2 + 2 hcl æ 2 h2o + 1 pbcl2 6) 2 albr3 + 3 k2so4 æ 6 kbr + 1 al2(so4)3 7) 1 ch4. (3) answer the questions related to each chemical formula. 2 fe + 3 h. Write the word equations below as chemical equations and balance:

![49 Balancing Chemical Equations Worksheets [with Answers] Worksheet](https://i2.wp.com/templatelab.com/wp-content/uploads/2017/01/balancing-equations-04.jpg)